
- BIONOTE
- Support
- News
-
 Bionote, Listed on KOSPI (Korea Composite Stock Price Index) as of 22nd of December
Bionote, Listed on KOSPI (Korea Composite Stock Price Index) as of 22nd of DecemberDISCLAIMER: This news article is a direct translation of the original Korean article from MTN. BioNote Inc. does not claim any right to the article or claim any ownership.The translation was completed by BioNote Inc. only for the convenience of non-Korean speaking customers and BioNote Inc. does not seek to redistribute nor acquire profit from the translation.Bionote, a veterinary diagnostics and biological raw materials company, has been listed on KOSPI, the Korea Composite Stock Price Index, as of 22nd of December.At the Listing Event held at the Korea Exchange on the 22nd, DVM Byung-Ki Cho (CEO of Bionotoe), Jae-Jun Lim (Head of Korea Exchange Securities Market Division), Young-Chae Jeong (CEO of NH Investments & Securities), Il-Moon Jeong (CEO of Korea Investments & Securities) and Nam-Ki Chae (President of Korea IR Service) was in attendance.Bionote, founded in 2003, is a affiliate company of SD Biosensor and has become one of the world's leading veterinary diagnostics and biological raw materials company representing Korea, through the accumulation of proprietary R&D and know-how.Based on the company's unique genetic recombinant antigen/antibody development technical ability and manufacturing technology, it stands out in the immunodiagnostics field. Furthermore, the company can implement all processes from raw material manufacturing to finished product manufacturing through its proprietary technologies.Bionote's KOSPI listing is expected to be a giant leap towards becoming top 3 in the world in animal diagnostics and biological raw materials field. Increased brand recognition, advantageous positionings in M&A negotiations and recruitment of talented individuals are just some of the advantages of being listed on KOSPI, through which Bionote will continue to accelerate the expansion of the company and its businessEarlier, CEO of Bionote, DVM Byung-Ki Cho, strongly emphasized that he will personally make sure to fulfill Bionote's responsibilities and obligations to its investors as well as share the fruits of corporate growth with its investors.Link to original article: https://news.mtn.co.kr/news-detail/2022122208163498203
- 2022.12.23
- Hits : 8,485
-
 Bionote Receives '300 Million USD Export Tower', a Commemorative Trophy from Korean MOTIE and KITA, a First for Animal Diagnostic Device Company
Bionote Receives '300 Million USD Export Tower', a Commemorative Trophy from Korean MOTIE and KITA, a First for Animal Diagnostic Device CompanyBionote has received the '300 million USD Export Tower' commemorative trophy. This is the second time that Bionote has received an export achievement trophy following last year's 100 million USD export commemorative trophy and it is the first time an animal diagnostic device company has received them.Every 5th of December (Official Day of Trade), the Korean Ministry of Trade, Industry and Energy (MOTIE) and the Korean International Trade Association (KITA) holds a ceremony where they select and rewards companies that have contributed to export performance through pioneering overseas markets and creating jobs, amongst other criteria. Bionote has over 160 sales networks in over 90 countries around the world, through which Bionote exports their products such as rapid diagnostic kits, fluorescence immunoassay products (Vcheck F) and ELISA kits, amongst many others. Furthermore, Bionote has subsidiaries in key countries with large diagnostic markets such as US and China, rapidly expanding Bionote's market share.Bionote's main diagnostic device, Vcheck F, is especially well received both domestically and internationally due to its high sensitivity, achieved through the use of Europium as a fluorescence material, and its capability to quantitatively measure diverse biomarkers. Bionote also expects to grow through expansion into new markets with their Vcheck M. POC Molecular Diagnostic Device, and Vcheck C, POC Clinical Chemistry Analyzer which are planned to be launched relatively soon.
- 2022.12.20
- Hits : 6,042
-
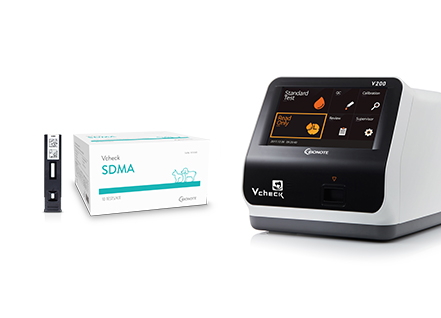 Bionote receives approval from MAFF Japan for sale of kindey disease diagnostic kit... "Will provide to 3,000 Japanese animal hospitals from next year onwards"
Bionote receives approval from MAFF Japan for sale of kindey disease diagnostic kit... "Will provide to 3,000 Japanese animal hospitals from next year onwards"DISCLAIMER: This news article is a direct translation of the original Korean article from The Stock. BioNote Inc. does not claim any right to the article or claim any ownership.The translation was completed by BioNote Inc. only for the convenience of non-Korean speaking customers and BioNote Inc. does not seek to redistribute nor acquire profit from the translation.Bionote, an in vitro diagnostic product manufacturer, announced they have completed the Japanses MAFF approval process for their 'Vcheck SDMA' kit, which is used for early screening of decreased kidney function in companion animals. Through this approval, it has become possible for Bionote to sell the 'Vcheck SDMA' kits in Japan, and Bionote has announced they will start providing it through their local Japanese partners.SDMA (Symmetric Dimethylarginine), is a amino acid that is produced during the process of breaking down proteins in the body and 90% of it is filtered by the kidney. If the kidney is not functioning properly, the SDMA does not get filtered out of the system and remains in the body. An IVD kit can be used to measure the SDMA levels in the body to screen for decreased kidney function and SDMA has less interfering factors than traditional biomarkers, with SDMA levels increasing after only 25 - 40% of damage to the kidneys.Traditional biomarkers that are used to test for kidney function, such as creatinine, is affected by factors such as whether the cat or dog in question has a lot of muscle mass and only starts to increase after around 75% of the kidney has been damaged. As such, in recent years, SDMA tests have been used more frequently for early screening of chronic kidney diseases, according to Bionote.Bionote's 'Vcheck SDMA' diagnostic kit quantitavely measures the SDMA amount in samples, using an analyzer called Vcheck F V200, quickly and with ease. Bionote also explained that when the Vcheck SDMA results were compared with lab tests, 98% of the results were in agreement. The kit only requires 100 microliters of serum or plasma (heparin) samples and the results can be confirmed after only 11 minutes, said the Bionote spokesperson.CEO of Bionote, Dr. Byung Ki Cho, said that "it has become possible for us to sell our Vcheck SDMA in Japan through this approval and registration" and that "We expect to be able to provide this product to over 3,000 animal hospitals in Japan from 2023 onwards". Bionote is currently in the process of going public in KOSPI through NH Investment & Securities and Korea Investment & Securities, at the end of the year.Link to original article: https://www.the-stock.kr/news/articleView.html?idxno=17476
- 2022.12.01
- Hits : 16,356